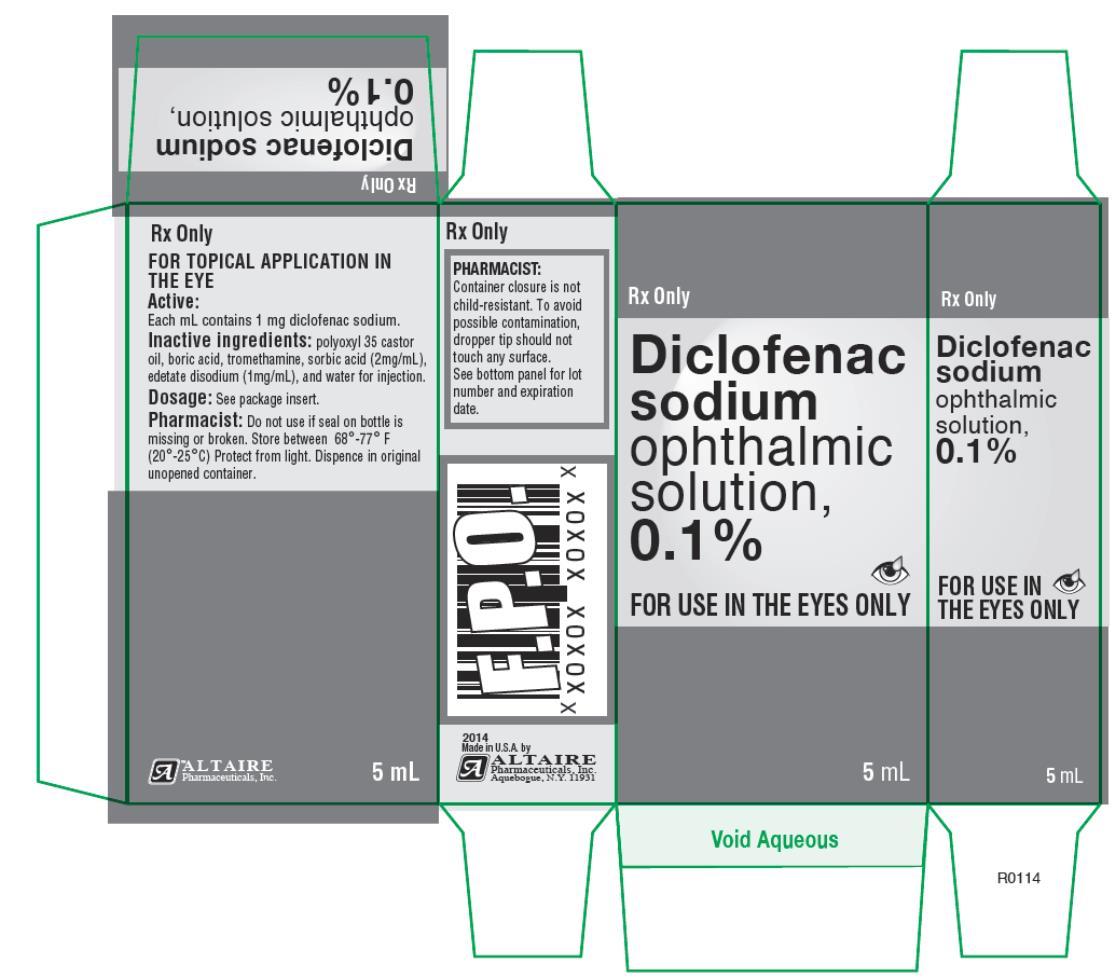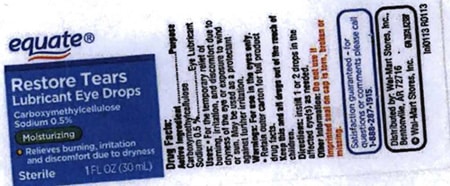
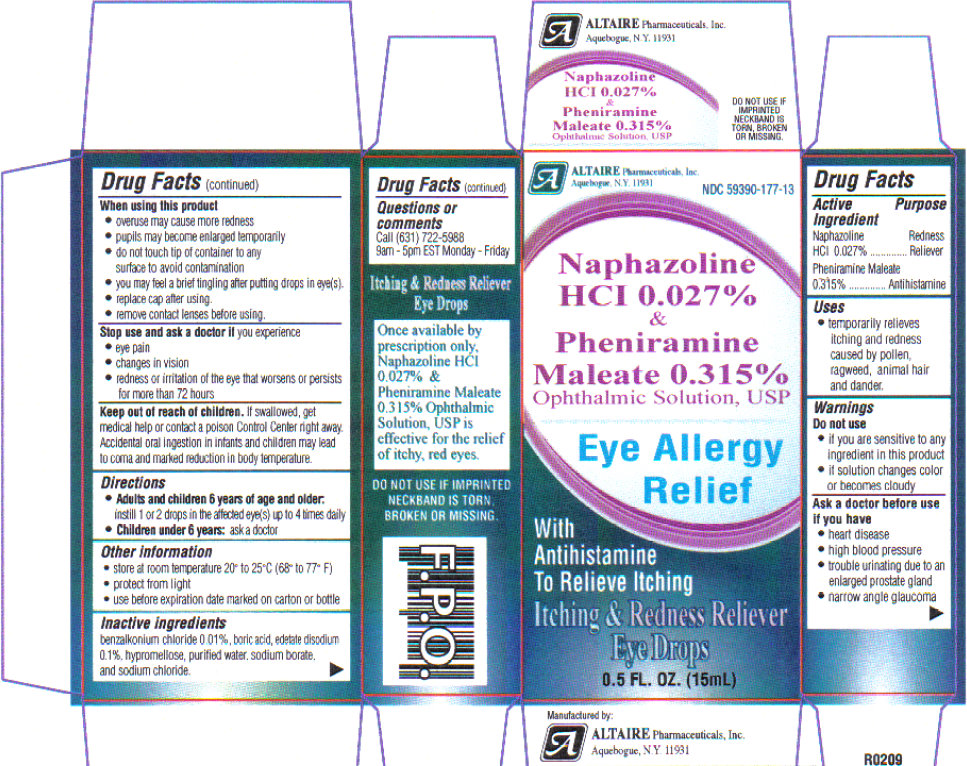
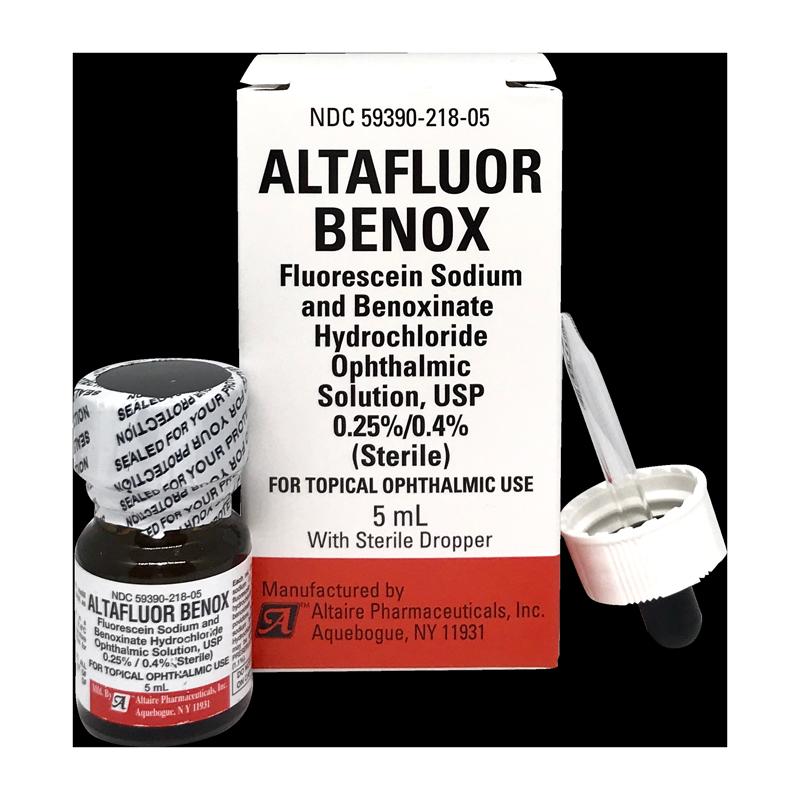
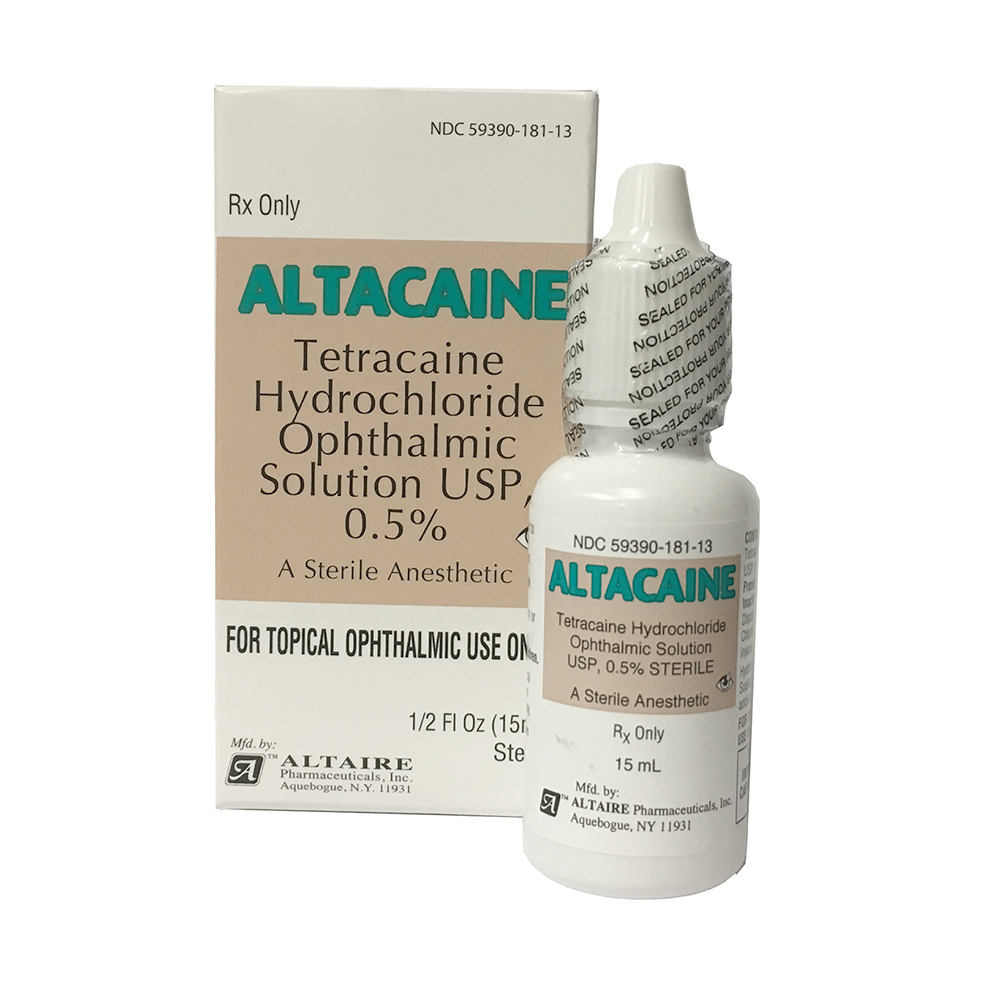
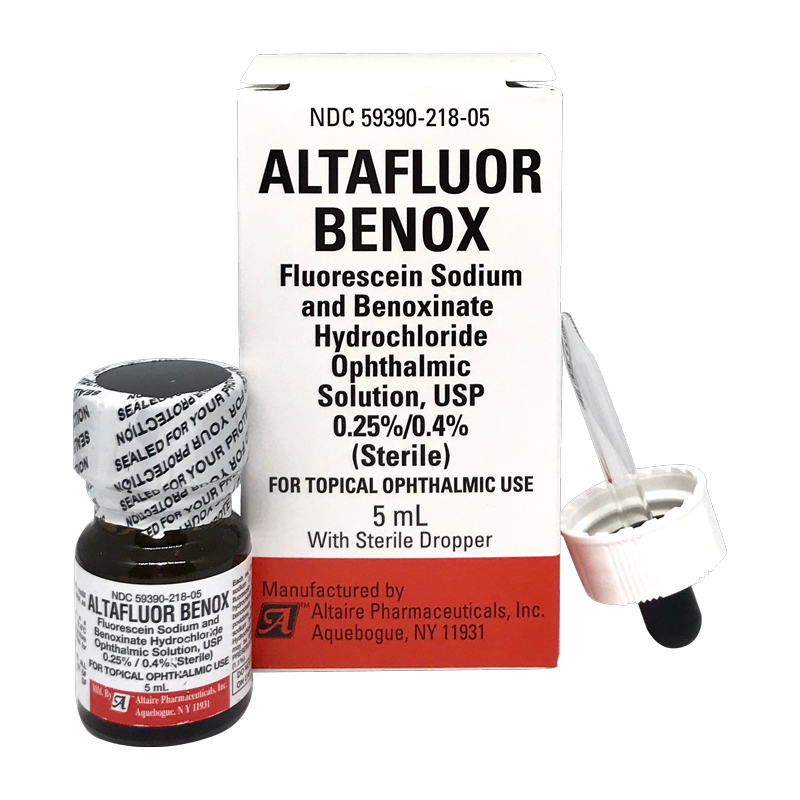
Altaire Pharmaceuticals Recalls Nine Lots of Eye Drops Due to Concerns About Preservative - Newsome | Melton Law

UPDATE: Massive Altaire recall sweeps 99 eyecare products off shelves - American Academy of Ophthalmology

Some eye drops, ointments sold at Walmart, Walgreens recalled because they might not be sterile: FDA - ABC News

Altaire Pharmaceuticals, Inc. Issues Voluntary Recall Of Multiple Eye Care Products Sold At CVS - Ksst Radio









/cloudfront-us-east-1.images.arcpublishing.com/gray/4DL5VLH45RFFDGGP575GMPAZGU.jpg)