Biological evaluation of medical devices - Standard 10993-7 - Laboratoire d'analyse, d'étude et expertise chimique
.jpg)
ANSI/AAMI/ISO 10993-7:2008 (R2012) - Biological evaluation of medical devices - Part 7: Ethylene oxide sterilization residuals

Thoughts on amendments to ISO 10993-7 medical device ethylene oxide sterilization residuals - Pharmaceutical Technology

SIST EN ISO 10993-7:2009/AC:2010 - Biological evaluation of medical devices - Part 7: Ethylene oxide

DTI Bureau of Philippine Standards - The Department of Trade and Industry's Bureau of Philippine Standards (DTI-BPS) informs concerned sectors that the following standards have been adopted and approved as Philippine National

ISO 10993-7 Biological Evaluation of Medical Devices - Test Standard for Ethylene Oxide Sterilization Residues

ISO 10993-7:2008, Second Edition: Biological evaluation of medical devices - Part 7: Ethylene oxide sterilization residuals: International Organization for Standardization: 9789267108506: Amazon.com: Books

Amazon.com: ANSI/AAMI/ISO 10993-7:2008/(R)2012 Biological evaluation of medical devices - Part 7: Ethylene oxide sterilization residuals : Industrial & Scientific

UNE EN ISO 10993-7:2009/A1:2022 Biological evaluation of medical devices - Part 7: Ethylene oxide sterilization residuals - Amendment 1: Applicability of allowable limits for neonates and infants (ISO 10993-7:2008/Amd 1:2019)

BS EN ISO 10993-7:2008+A1:2022 - TC Tracked Changes. Biological evaluation of medical devices Ethylene oxide sterilization residuals
Biocompatibility & analysis of medical devices according to ISO 10993 - ISO 10993-7: Ethylene oxide sterilization residuals - Danish Technological Institute

ISO 10993-7:2008 - Biological evaluation of medical devices — Part 7: Ethylene oxide sterilization residuals




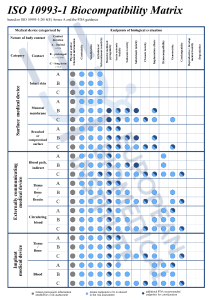
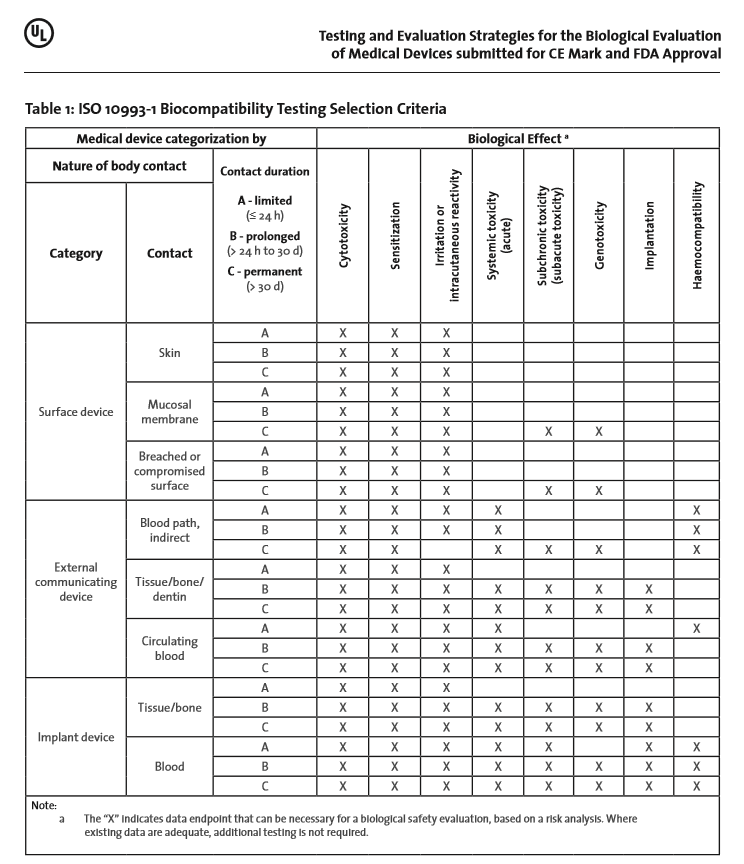
2001.jpg)





