Gastro-resistance of pantoprazole microparticles and agglomerates (A to... | Download Scientific Diagram

Terrace Pharmaceuticals Pvt.Ltd. - Penzolin- O Pantoprazole and Ondansetron Tablets Pantoprazole is used to treat the following conditions: Barrett's Esophagus. Dumping Syndrome. Duodenal Ulcer. Erosive Esophagitis. Gastritis/Duodenitis. GERD ...

On the Mechanism of Formation and the Synthesis of Pantoprazole Sodium Sesquihydrate-Related Compound E: A Phantom Chemical Entity | ACS Omega

On the Mechanism of Formation and the Synthesis of Pantoprazole Sodium Sesquihydrate-Related Compound E: A Phantom Chemical Entity | ACS Omega
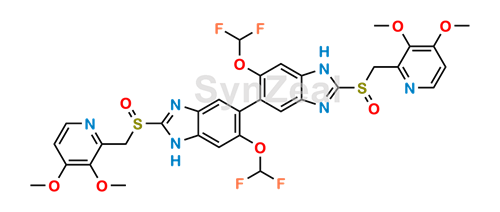
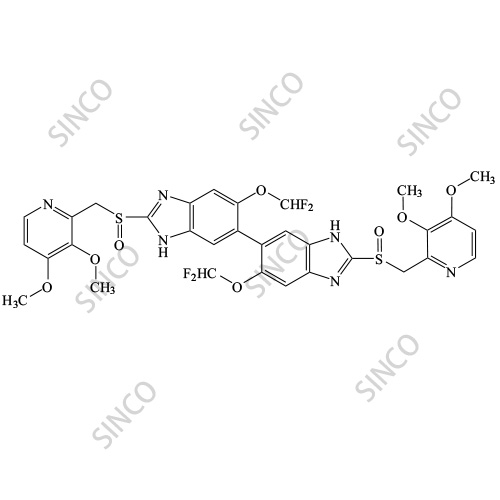
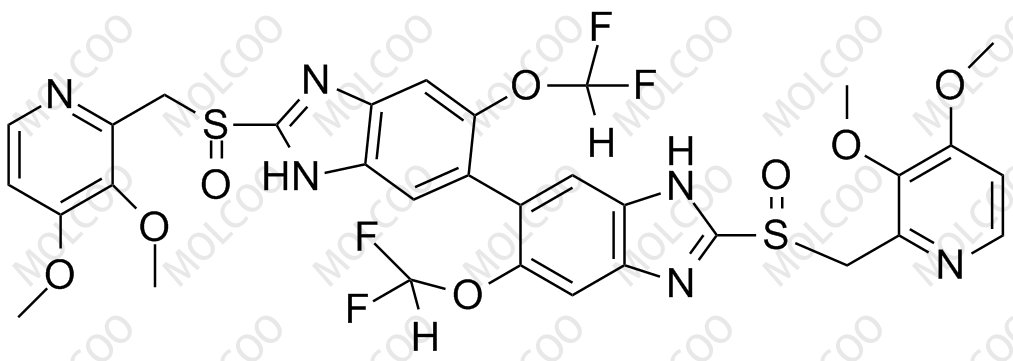
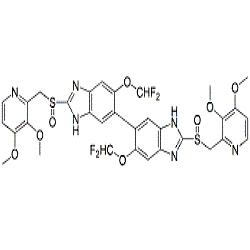
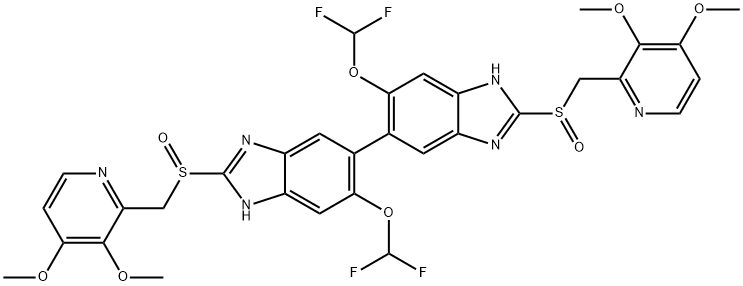
![2115779-15-0 | Pantoprazole Dimer (>90%) | 6,6'-Bis(difluoromethoxy)-2,2'-bis(((3,4-dimethoxypyridin-2-yl)methyl)sulfinyl)-3H,3'H-5,5'-bibenzo[d]imidazolel; USP Pantoprazole Related Compound E; | C₃₂H₂₈F₄N₆O₈S₂ | TRC 2115779-15-0 | Pantoprazole Dimer (>90%) | 6,6'-Bis(difluoromethoxy)-2,2'-bis(((3,4-dimethoxypyridin-2-yl)methyl)sulfinyl)-3H,3'H-5,5'-bibenzo[d]imidazolel; USP Pantoprazole Related Compound E; | C₃₂H₂₈F₄N₆O₈S₂ | TRC](https://www.trc-canada.com/prod-img/P182900.png)
2115779-15-0 | Pantoprazole Dimer (>90%) | 6,6'-Bis(difluoromethoxy)-2,2'-bis(((3,4-dimethoxypyridin-2-yl)methyl)sulfinyl)-3H,3'H-5,5'-bibenzo[d]imidazolel; USP Pantoprazole Related Compound E; | C₃₂H₂₈F₄N₆O₈S₂ | TRC

FIGURE E Time vs. Concentration curves for intravenous (IV; square) and... | Download Scientific Diagram

e Chemical structures of (A) rabeprazole, (B) pantoprazole, and (C)... | Download Scientific Diagram
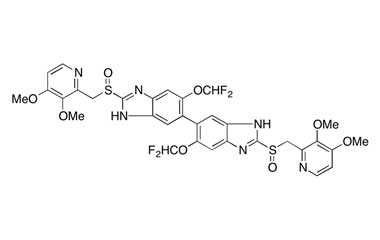
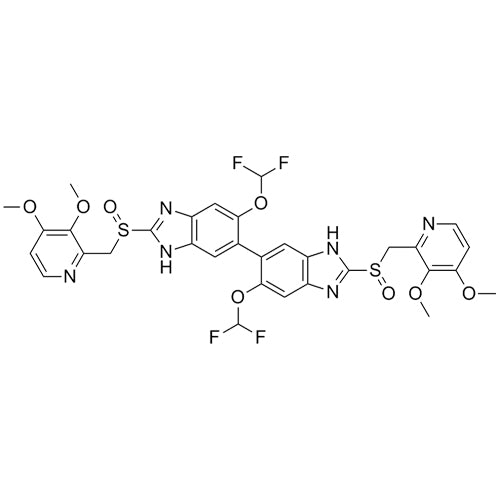
CAS No : 2115779-15-0| Product Name : Pantoprazole - Impurity E| Chemical Name : Pantoprazole Dimer | Pharmaffiliates

Biological E. Limited - We are proud to announce that we have received the final USFDA approval on an Abbreviated New Drug Application (ANDA), Pantoprazole Sodium, for injections of 40 mg per